Product Details
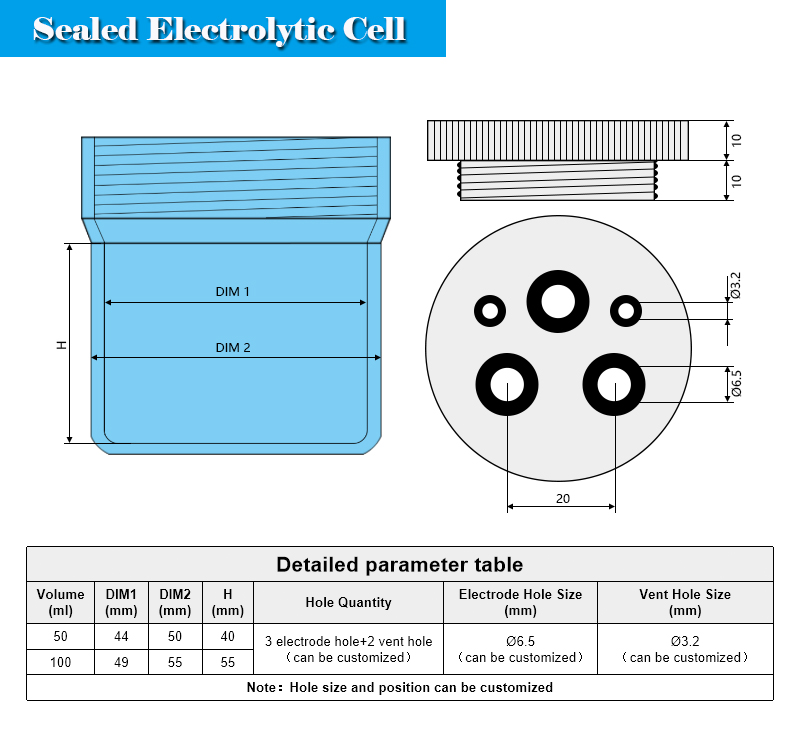
Conventional electrochemical cells include ordinary electrolytic cells, sealed electrolytic cells, traditional five-port electrolytic cells, and PTFE electrolytic cells. If the measurement needs to be carried out in a certain gas atmosphere or an oxygen-free environment, a sealed electrolytic cell must be used and equipped with air inlet and outlet pipes.
The sealed electrolytic cell is composed of a high borosilicate glass cell body and a PTFE cover. The tank body and the cover are connected by threads, and the fluorine rubber O-ring is embedded in the cover at the connection. The sealed electrolytic cell generally has three sealed electrode holes and two sealed air holes, all of which are sealed and fixed by sealing screws and fluorine rubber O-rings. The lower glass tank body can also be customized into a double-jacketed glass type according to the temperature control requirements. It is connected to the outer tank body through the hose of a peristaltic pump or a circulating pump. The liquid circulates in the glass jacket layer。 Relying on the exchange of cold and heat to achieve the effect of cooling or heating the electrolyte solution.
When using a sealed electrolytic cell, the air inlet and outlet operations are generally performed before or during the experiment, and a glass tube with a glass filter element at the end is selected to be inserted into the lower end of the air inlet hole. The gas can be converted into small bubbles for uniform aeration, which can fully drive the remaining bubbles in the liquid, and can also dissolve the electrolyte more fully, and avoid the accumulation of large bubbles from interfering with the working electrode test. Although the reference electrode and the working electrode are in the same chamber, the user can also choose to match the bent Luggin capillary as the built-in salt bridge. The lower end of the salt bridge faces the working electrode, so that the distance between the reference electrode and the working electrode can be reduced, the effect of R drop is effectively reduced, and the resistance overpotential is eliminated. Filling fluid needs to be added to the salt bridge, and the concentration of the filling fluid must be the same as the filling fluid of the inserted reference electrode.
The volume of the electrolytic cell should be selected moderately. If the volume is too large, the amount of solution will be too much, which is unnecessary. During electrochemical measurement, the electrode surface undergoes oxidation and reduction reactions, and some substances in the solution are reduced by participating in the electrode reaction. At the same time, some reaction products will dissolve into the solution. If the volume of the electrolytic cell is too small, the solution concentration will change significantly during the long-term steady-state measurement, which will eventually affect the experimental results. In most cases, the volume of 50ml and 100ml are more choices.
The material of the electrolytic cell must have good stability. In general, high borosilicate glass is widely used to make electrolytic cells of various shapes because of its good chemical stability, wide use temperature, high transparency and processing performance. The electrolytic cell made of glass is very stable in most inorganic electrolyte solutions and organic electrolyte solutions. At the same time, considering the use requirements of some special electrolyte systems and special shapes, PTFE, plexiglass, PEEK materials and various types of metal materials can also be used as preparation materials for electrolytic cells.
Product Includes: 1 * PTFE lid 1 * chamber 6 * electrode hole sealing screw 4 * gas hole sealing screw 1 * curved luggin salt bridge 1 * glass filter aeration pipe 1 * PTFE tube φ3mm 15 * fluorine rubber O-ring
References:
NEED HELP? CONTACT US
Call Toll-Free +1 (800) 972-7086
Email Us:
- Shipping
- Standard Shipping: 5-7 Business days
- DHL/Fedex Express Shipping: 2-5 Business days
PAYMENT
PRODUCT SHOW
Here are some photos, you can find the details from them, if you need more information, you can talk with us, we have many experts in dekresearch, and we are very happy to give you some support in your items!




We devotes itself to providing laboratory instrumentation that enables simultaneous in-situ measurements of a number of signals (electrochemical, optical, thermal and other) on thin film and membrane materials. we have already gained so much experience in the field of electrochemical trading and our final goal is to offer our customers various kinds of laboratory instrumentation and at best price. We are gradually becoming one of the top instrumentation online shop throughout the world and we do hope we could make you outstanding by providing the real good stuff you want. Enjoy your shopping with us and have a nice day.

 United States (USD)
United States (USD) United Kingdom (GBP)
United Kingdom (GBP) Canada (CAD)
Canada (CAD) Singapore (SGD)
Singapore (SGD) New Zealand (NZD)
New Zealand (NZD) Ireland (EUR)
Ireland (EUR) India (INR)
India (INR)